A research team from the Hong Kong University of Science and Technology (HKUST) has identified a key protein that may shed light on how to reverse the aging process using the adult stem cell of skeletal muscle (or muscle stem cells, MuSCs) as a model system. The discovery paves the way for the possible future development of therapeutic interventions for aging-related diseases and various mitochondrial diseases.
During human aging, cells undergo a process of cellular senescence, where they no longer divide but do not die, acting like "zombie cells," accumulating in the human body, causing cellular damage, and contributing to age-related defects. Importantly, the ability of cells to maintain healthy functions relies upon their capacity to generate chemical energy, of which mitochondria, the powerhouse of the cell, plays a vital role. However, as cells age, their ability to produce sufficient energy declines. The loss of mitochondrial activity has been associated with senescence in many tissues. Proper mitochondrial functions are important for MuSCs to repair damaged skeletal muscle following injury and to maintain the resident stem cell pool for future regeneration. Yet, the signaling pathways which regulate mitochondrial metabolism during aging remain unclear.
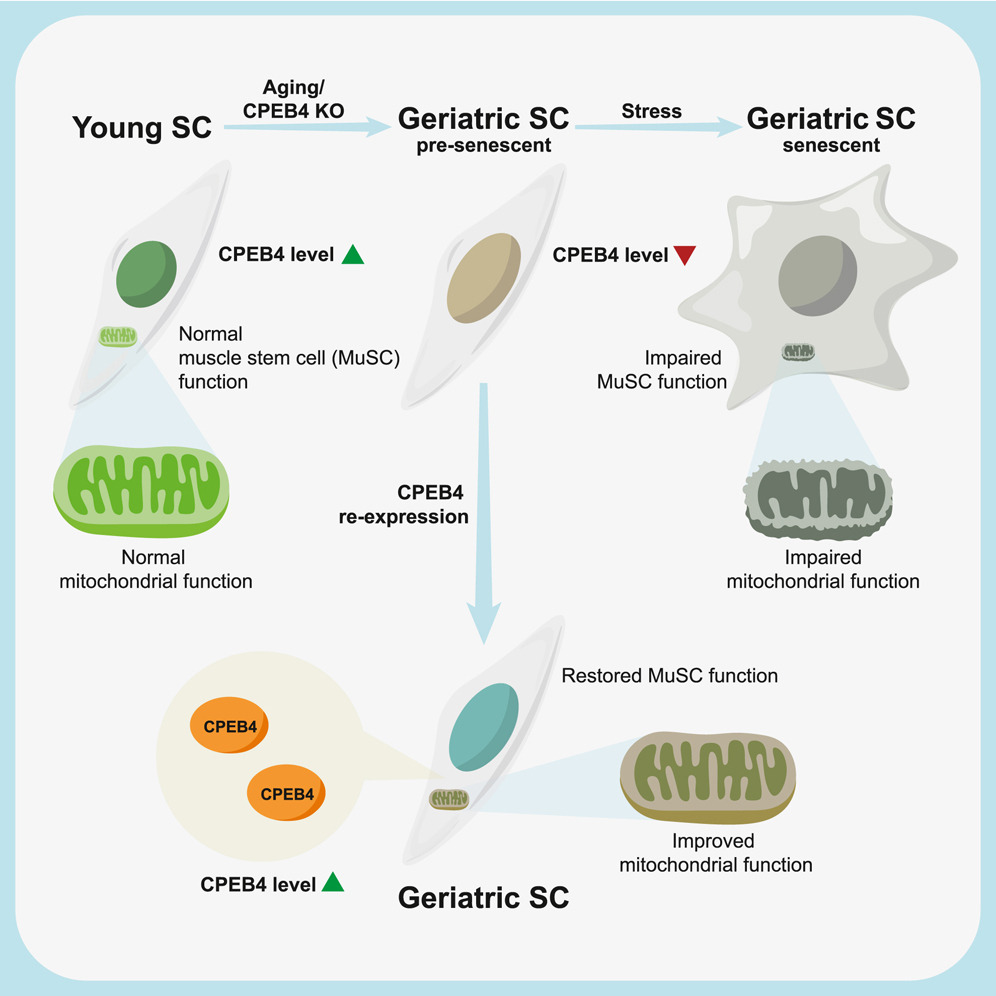
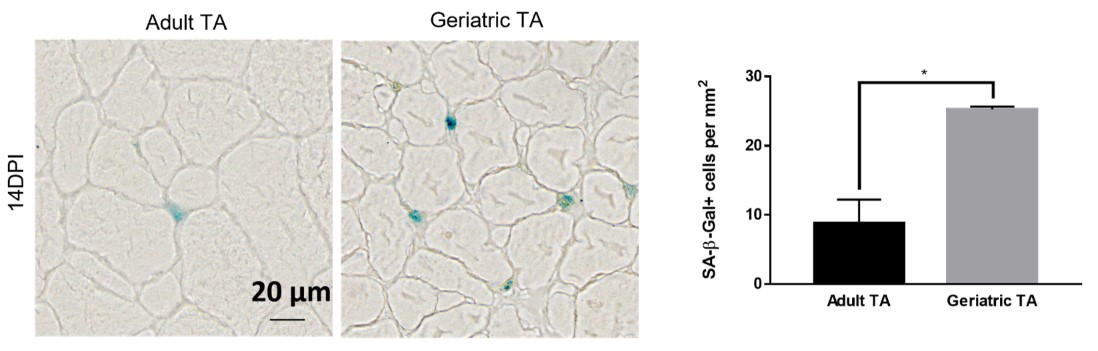
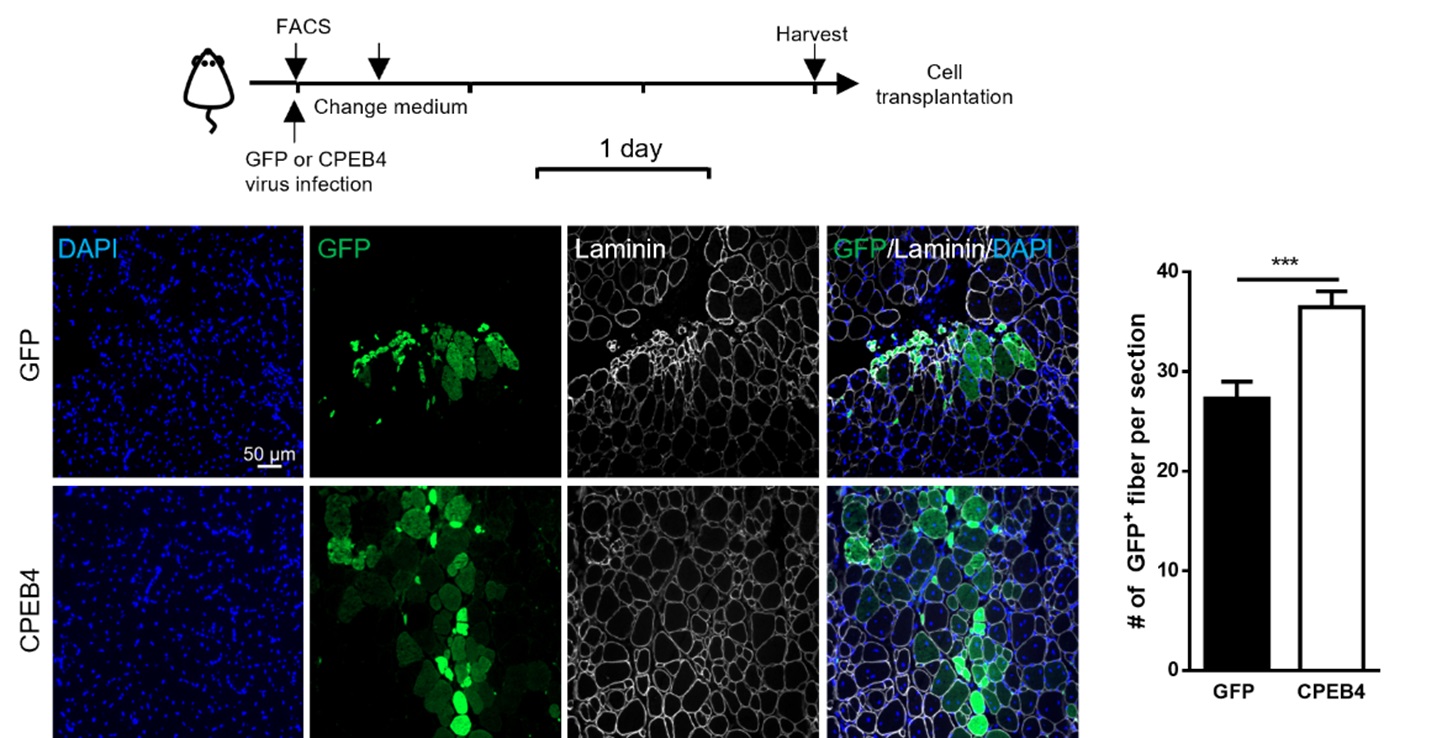
A laboratory group led by Prof. Tom CHEUNG, the S H Ho Associate Professor of Life Science in the Division of Life Science at HKUST, recently identified the role of CPEB4, an mRNA-binding protein, in maintaining mitochondrial metabolism, by upregulating the biosynthesis of mitochondrial proteins, thus maintaining sufficient energy output. In addition, the team showed that the levels of CPEB4 protein decreased in various aging murine tissues, particularly skeletal muscle. They also observed that aged muscle shows signs of senescence following muscle injury, as shown by the increased presence of the senescence marker, senescence-associated β-galactosidase (SA-β-gal), compared to adult muscle (see Figure 1). Importantly, their research demonstrated that restoring CPEB4 expression in aged MuSCs increased mitochondrial protein production, boosted energy production, and impressively protected against cellular senescence. Of note, the transplantation of CPEB4 re-expressing MuSCs into geriatric recipient mice led to improved muscle repair (see Figure 2). Similarly, CPEB4 expression in various human cell lines also protected against cellular senescence.
Prof. Cheung comments, "Our findings highlight the importance of maintaining mitochondrial functions and its proteome by mRNA-binding proteins in muscle stem cells. More importantly, our study provides new insights into the diagnostic and therapeutic potential of CPEB4 to rescue mitochondrial defects and reverse cellular senescence during aging. Our work also lays the groundwork to study further the feasibility of developing CPEB4 as a potential therapeutic target for various mitochondrial diseases such as Leigh Syndrome."
These findings were recently published in Developmental Cell.
More about HKUST